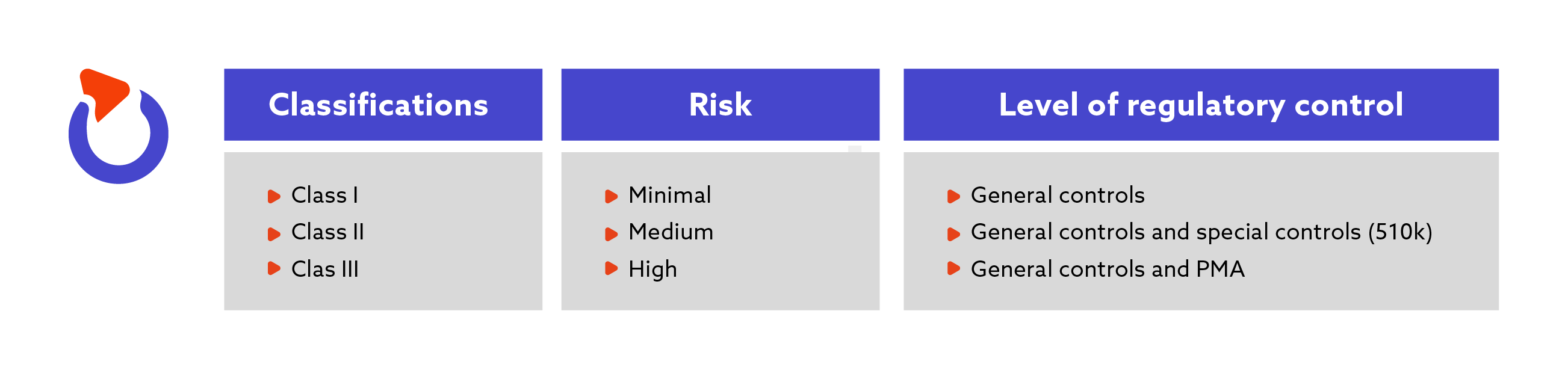
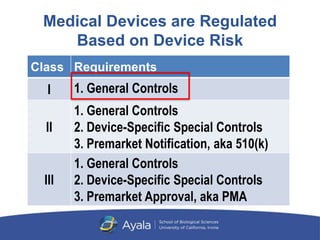
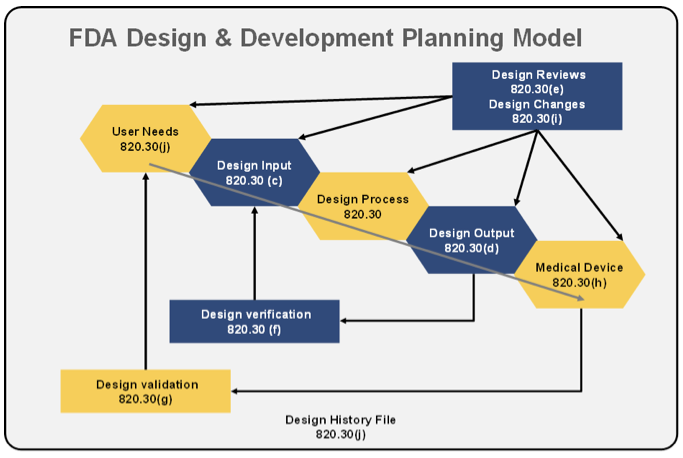
Current perspectives on the US FDA regulatory framework for intelligent drug-delivery systems | Therapeutic Delivery
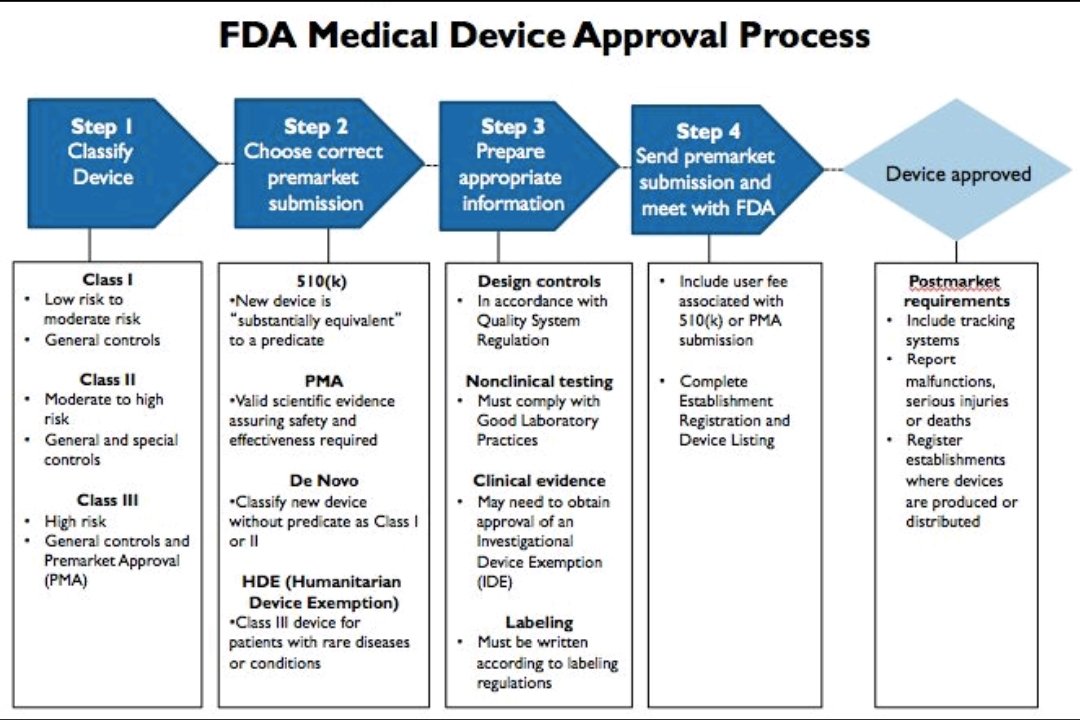
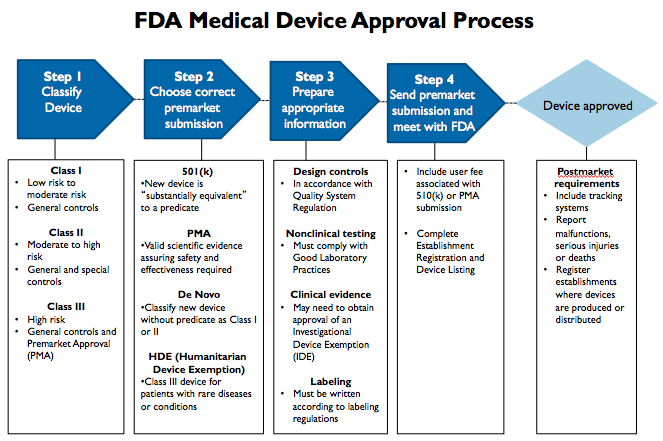
Dr Kalpesh hegde on X: "FDA Medical devices approval process in 5 steps. #medicaldevices #MDR #IVDR https://t.co/1GL295rEVO" / X
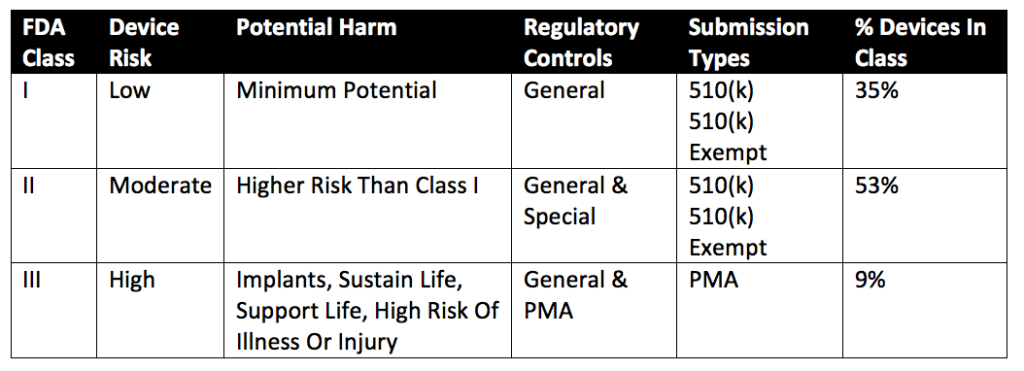
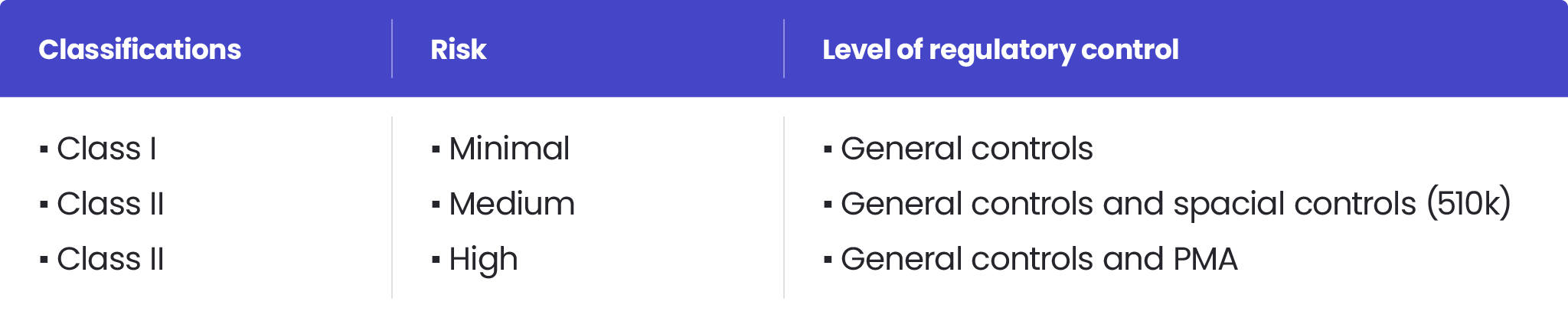
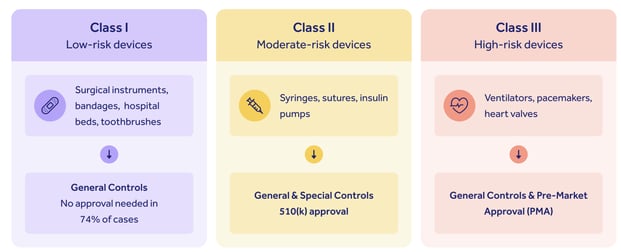
A comparison of the hierarchical structure of the regulation of medical... | Download Scientific Diagram